57+ calculate the ratio of effusion rates for 235u and 238u.
Molecular Effusion and Diffusion Last updated Save as PDF Page ID 21766 Learning. Web Calculate the ratio of effusion rates for 235U and 238U and compare it to the ratio for UF6 given in the essay.
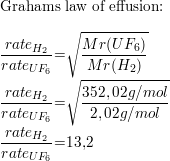
What Is The Ratio Of Effusion Rates For The Lightest Gas H2 Quizlet
The atomic masses are 235U 23504 amu.
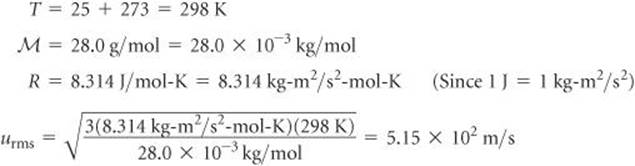
. Web Calculate The Ratio Of Effusion Rates For 235u And 238u This is likewise one of the factors by obtaining the soft documents of this calculate the ratio of effusion. The atomic mass of u-235 is 235054 amu and that of u-238 is 238051 amu. 1086 As discussed in the.
Web Calculate the ratio of rates of effusion of 235 UF 6 and 238 UF 6 where 235 U and 238 U are isotopes of uranium. Web Calculate the ratio of effusion rates for 238uf6 and 235uf6. Web Calculate the ratio of rates of effusion of 235UF6 and 238UF6 where 235U and 238U are isotopes of uranium.
Web calculate the ratio of effusion rates for 235u and 238u is available in our book collection an online access to it is set as public so you can get it instantly. Web Calculate the molar masses of 235 UF 6 and 238 UF 6 and then use Grahams law to determine the ratio of the effusion rates. Web Calculate the ratio of effusion rates for 238uf6 and 235uf6.
Aug 28 2022Part a calculate the ratio of effusion rates for 238 uf6 and 235 uf6. Web Calculate The Ratio Of Effusion Rates For 235U And 238U. The atomic mass of U-235 is 235054 amu and that of U-238 is 238051 amu.
Web Calculate the ratio of rates of effusion of 235UF6 and 238UF6 where 235U and 238U are isotopes of uranium. Web The ratio of rates of diffusion of gases X and Y is 15 and that of Y and Z is 16. Web Calculate the ratio of effusion rates for 238UF6 and 235UF6.
View solution The rates of diffusion of two. Web Calculate The Ratio Of Effusion Rates For 235U And 238U. Isotopic content of naturally occurring uranium and atomic masses of 235u and 238u asked for.
The atomic weights are 235 U 23504 amu. Question Transcribed Image Text. What is the ratio in the form of a decimal of the RMS.
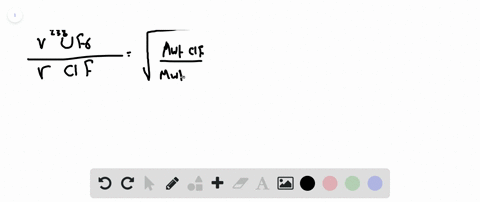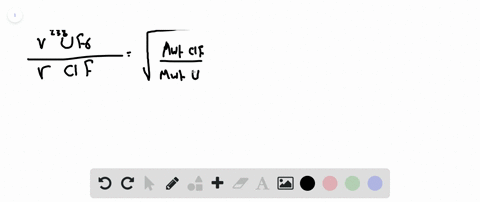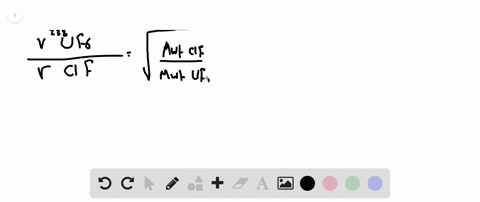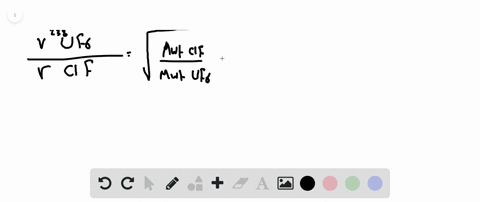
Web The two isotopes of uranium 238U and 235U can be separated by effusion of the corresponding U F 6 gases. Use this value to determine the isotopic. The atomic mass of u-235 is 235054 amu and that of u-238 is 238051 amu.
The ratio of rate of diffusion of Z and X is. The atomic weights are 235U 23504 amu.
Solved Calculate The Ratio Of Diffusion Rates For Carbon Monoxide And Carbon Dioxide

Solved We Separate U 235 From U 238 By Fluorinating A Sample Of Uranium To Form Uf 6 Which Is A Gas And Then Taking Advantage Of The Different Rates Of Effusion And Diffusion

Calaulate Relative Rates Of Effusion Of O2 To Ch4 Through A Container Containing O2 And Ch4 In 3 2 Mass Ratio
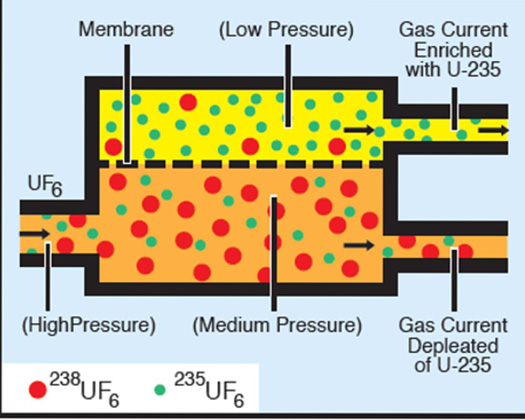
9 Uranium Enrichment Chemistry Libretexts

Solved Calculate The Ratio Of Rates Of Effusion Of 235 Uf6 And 238 Uf6 Where 235 U And 238 U Are Isotopes Of Uranium The Atomic Masses Are 235 U 235 04 Amu
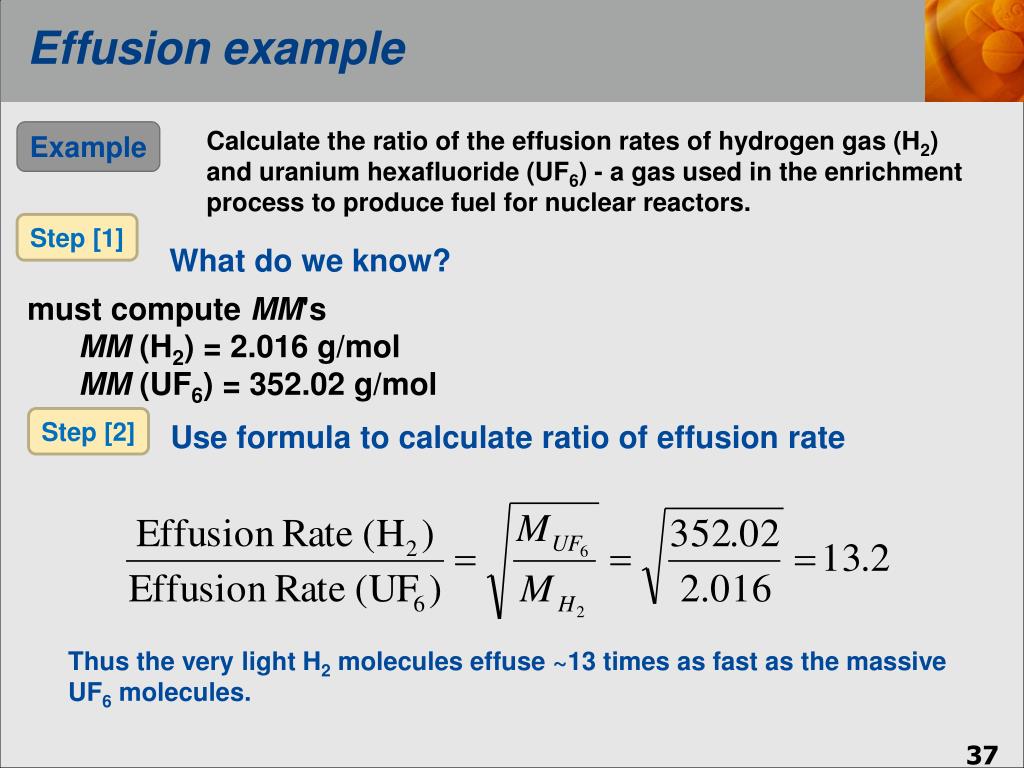
Ppt Chapter 11 Properties Of Gasses Powerpoint Presentation Free Download Id 4666732
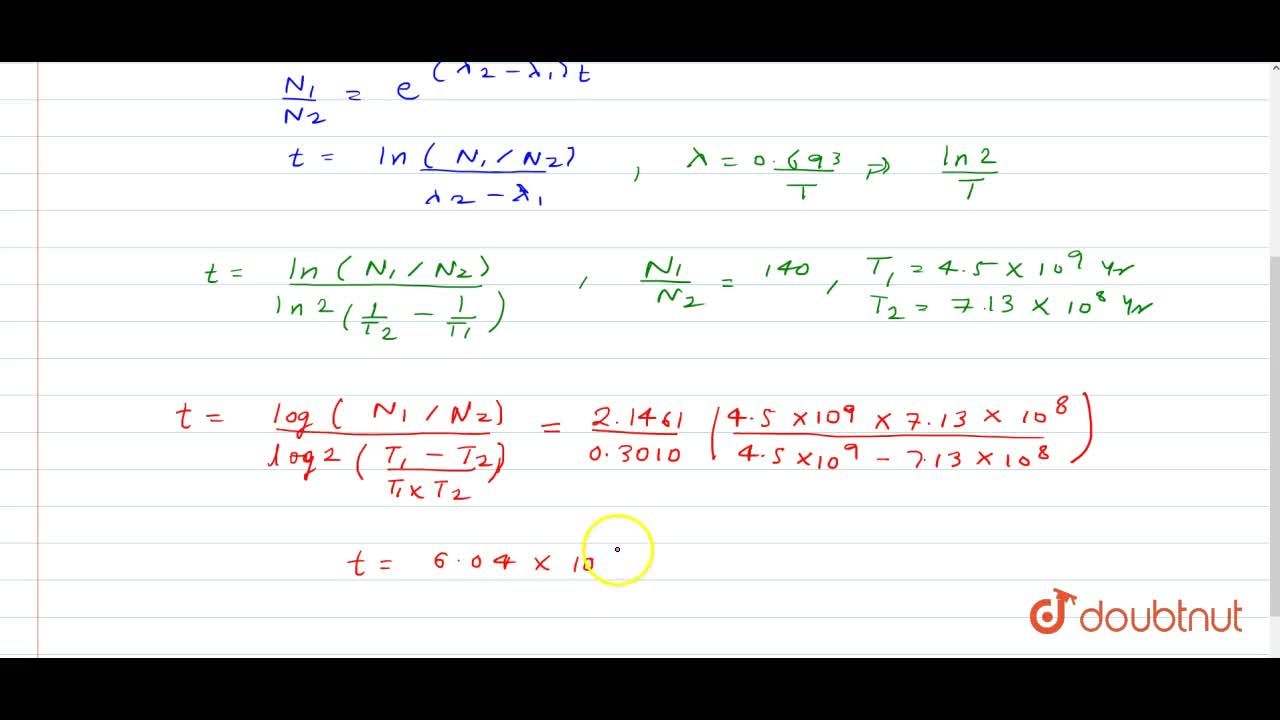
The Isotope Of U 238 And U 235 Occur In Nature In The Ratio 140 1 Assuming That At The Time Of Earth S Formation They Were Present In Equal Ratio Make An Estimate Of The

Calculate The Relative Rates Of Diffusion Of 235uf6 And 238uf6 In The Gaseous State At Mass Of F 19
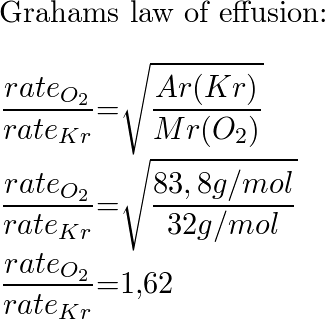
Solved Calculate The Ratio Of Effusion Rates For Ar And Kr

Solved One Way Of Separating Oxygen Isotopes Is By Gaseous Diffusion Of Carbon Monoxide The Gaseous Diffusion Process Behaves Like An Effusion Process Calculate The Relative Rates Of Effusion Of 12 C 16 O
What Is The Ratio Of Effusion Rates For O2 And Kr Quizlet

1 Polonium Metal Po Has A Simple Cubic Unit Cell A Sketch The Cell B Calculate The Number Of Points Atoms Per Cell And C If The Distance Between The Nearest Neighbors

Openstax College Physics Solution Chapter 13 Problem 48 Problems Exercises Openstax College Physics Answers
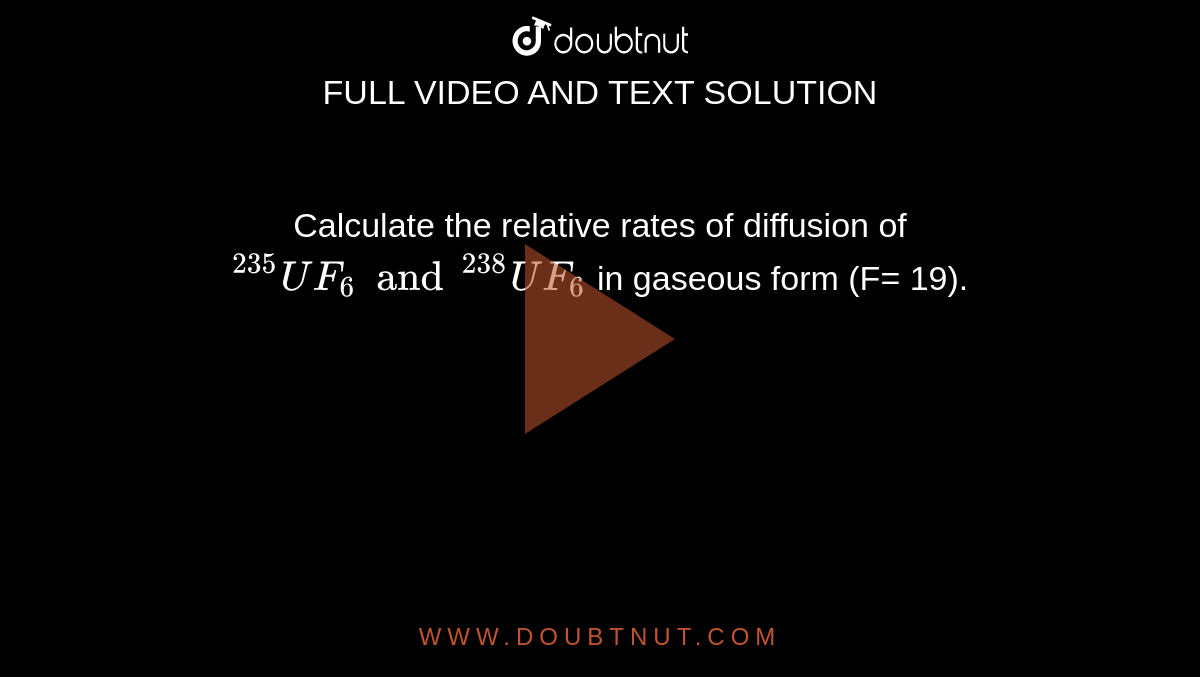
Calculate The Relative Rates Of Diffusion Of 235uf6 And 238uf6 In Gaseous Form F 19
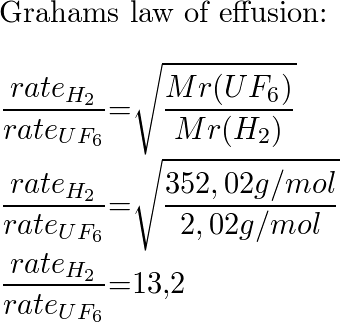
What Is The Ratio Of Effusion Rates For The Lightest Gas H2 Quizlet

Solved Calculate The Ratio Of Effusion Rates For Ar And Kr
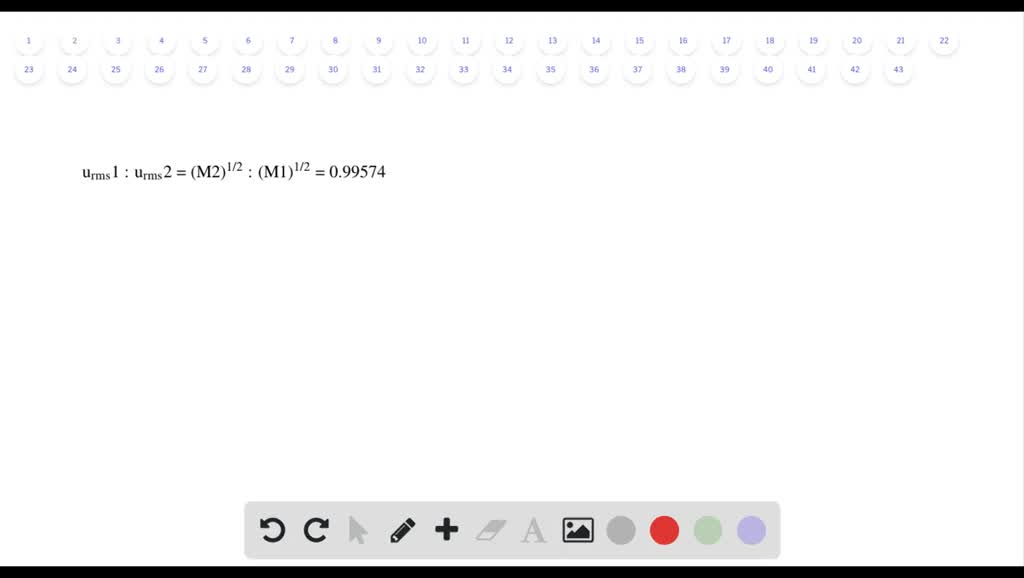
Solved We Separate U 235 From U 238 By Fluorinating A Sample Of Uranium To Form Uf 6 Which Is A Gas And Then Taking Advantage Of The Different Rates Of Effusion And